Fiber Photometry System
RECORD REAL-TIME FLUOROPHORE RESPONSES WITH THE RZ10X
Description
Specs & Resources
Publications
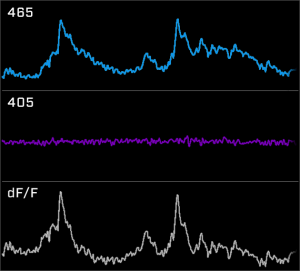
Fiber photometry is an easy way to detect neural activity using fluorescent proteins. Researchers can use genetic animal models and fluorescent proteins, such as calcium (GCaMP) and dopamine (dLight), to study specific brain circuits.
TDT’s Fiber Photometry System is an out-of-the-box solution designed for researchers. TDT’s user-friendly Synapse software provides a seamless interface to the real-time LUX RZ10X processor. Integrated and swappable LUX LEDs and photosensors streamline hardware setup and debugging. TDT’s systems allow you to focus on your research, not equipment.
Synapse and our LUX hardware simplify your fiber photometry research. With Synapse, record and view your fluorophore responses in real-time while behavior and video data is automatically time-locked. Optogenetics and electrophysiology can also be incorporated for multi-modal and closed-loop experiments.
RZ10X Fiber Photometry User Guide
Offline Data Analysis for Fiber Photometry:
2022 * RZ10X
* Goldstein, … Betley, et al. (2022). “Specificity of Varenicline in Blocking Mesolimbic Circuit Activation to Natural and Drug Rewards”. Neuroscience, 483. https://doi.org/10.1016/J.NEUROSCIENCE.2021.12.016
Liu, … Anderson, et al. (2022). “Make war not love: The neural substrate underlying a state-dependent switch in female social behavior”. Neuron. https://doi.org/10.1016/j.neuron.2021.12.002
Wu, … Halpern, et al. (2022). “Local accumbens in vivo imaging during deep brain stimulation reveals a strategy-dependent amelioration of hedonic feeding”. Proceedings of the National Academy of Sciences, 119(1). https://doi.org/10.1073/pnas.2109269118
2021 * RZ10X
* Copits, … Bruchas, et al. (2021). “A photoswitchable GPCR-based opsin for presynaptic inhibition”. Neuron, 109(11). https://doi.org/10.1016/J.NEURON.2021.04.026
* Ferrara, … Rosenkranz, et al. (2021). “Developmental Shifts in Amygdala Activity during a High Social Drive State”. Journal of Neuroscience, 41(45). https://doi.org/10.1523/JNEUROSCI.1414-21.2021
* Horio, Liberles. (2021) “Hunger enhances food-odour attraction through a neuropeptide Y spotlight”. Nature, 592(7853). https://doi.org/10.1038/s41586-021-03299-4
Samineni, … Gereau, et al. (2021). “Cellular, circuit and transcriptional framework for modulation of itch in the central amygdala”. ELife, 10. https://doi.org/10.7554/eLife.68130
Robert, … Polley, et al. (2021). “A functional topography within the cholinergic basal forebrain for encoding sensory cues and behavioral reinforcement outcomes”. ELife, 10. https://doi.org/10.7554/eLife.69514
Reichenbach, … Andrews, et al. (2021). “Glucose-sensing in AgRP neurons integrates homeostatic energy state with dopamine signalling in the striatum”. BioRxiv. https://doi.org/10.1101/2021.03.22.436393
Tian, … Beier, et al. (2021). “An extended amygdala-midbrain circuit controlling cocaine withdrawal-induced anxiety and reinstatement”. BioRxiv. https://doi.org/10.1101/2021.11.05.467532
Chiacchierini, … McCutcheon, et al. (2021). “Protein Appetite Drives Macronutrient-Related Differences in Ventral Tegmental Area Neural Activity”. The Journal of Neuroscience, 41(23). https://doi.org/10.1523/JNEUROSCI.3082-20.2021
Choi, … McNally, et al. (2021). “A corticothalamic circuit trades off speed for safety during decision-making under motivational conflict”. BioRxiv. https://doi.org/10.1101/2021.11.21.469477
Feng, … He, et al. (2021). “The entorhinal cortex modulates trace fear memory formation and neuroplasticity in the lateral amygdala via cholecystokinin”. BioRxiv. https://doi.org/10.1101/2021.04.18.440346
Penzo, … Chudasama, et al. (2021). “Divergent projections of the paraventricular nucleus of the thalamus mediate the selection of reactive and proactive defensive behaviors”. Research Square. https://doi.org/10.21203/rs.3.rs-322756/v1
Ghareh, … Marchant, et al. (2021). “Role of anterior insula cortex in context-induced relapse of nicotine-seeking”. BioRxiv. https://doi.org/10.1101/2021.12.08.471717
Holly, … Fuccillo, et al. (2021). “Striatal low-threshold spiking interneurons locally gate dopamine”. Current Biology, 31(18). https://doi.org/10.1016/J.CUB.2021.06.081
Wang, … Pitt, et al. (2021). “Scn2a severe hypomorphic mutation decreases excitatory synaptic input and causes autism-associated behaviors”. JCI Insight, 6(15). https://doi.org/10.1172/jci.insight.150698
Luchsinger, … Centanni, et al. (2021). “Delineation of an insula-BNST circuit engaged by struggling behavior that regulates avoidance in mice”. Nature Communications, 12(1). https://doi.org/10.1038/s41467-021-23674-z
van Zessen, … Lüscher, et al. (2021). “Dynamic dichotomy of accumbal population activity underlies cocaine sensitization”. ELife, 10. https://doi.org/10.7554/eLife.66048
McGovern, … Root, et al. (2021). “Neurochemical Signaling of Reward and Aversion to Ventral Tegmental Area Glutamate Neurons”. The Journal of Neuroscience, 41(25). https://doi.org/10.1523/JNEUROSCI.1419-20.2021
Yau, … McNally, et al. (2021). “The Roles of Basolateral Amygdala Parvalbumin Neurons in Fear Learning”. The Journal of Neuroscience, 41(44). https://doi.org/10.1523/JNEUROSCI.2461-20.2021
Melchior, … Winder, et al. (2021). “Cocaine Augments Dopamine-Mediated Inhibition of Neuronal Activity in the Dorsal Bed Nucleus of the Stria Terminalis”. Journal of Neuroscience, 41(27). https://doi.org/10.1523/JNEUROSCI.0284-21.2021
Smiley, … Gass, et al. (2021). “Adolescent exposure to delta-9-tetrahydrocannabinol and ethanol heightens sensitivity to fear stimuli”. Behavioural Brain Research, 415. https://doi.org/10.1016/J.BBR.2021.113517
Zhang, … Ma, et al. (2021). “Ventral striatal islands of Calleja neurons control grooming in mice”. Nature Neuroscience, 24(12). https://doi.org/10.1038/s41593-021-00952-z
Thirtamara Rajamani, … Harony-Nicolas, et al. (2021). “Efficiency of cell-type specific and generic promoters in transducing oxytocin neurons and monitoring their neural activity during lactation”. Scientific Reports 2021 11:1, 11(1). https://doi.org/10.1038/s41598-021-01818-x
Martyniuk, … Kellendonk, et al. (2021). “Dopamine D2Rs Coordinate Cue-Evoked Changes in Striatal Acetylcholine Levels”. BioRxiv. https://doi.org/10.1101/2021.12.08.471871
He, … Xu, et al. (2021). “5-HT recruits distinct neurocircuits to inhibit hunger-driven and non-hunger-driven feeding”. Molecular Psychiatry. https://doi.org/10.1038/s41380-021-01220-z
Jung, … Kim, et al. (2021). “A forebrain neural substrate for behavioral thermoregulation”. Neuron. https://doi.org/10.1016/j.neuron.2021.09.039
Jean-Richard-dit-Bressel, … McNally, et al. (2021). “Instrumental aversion coding in the basolateral amygdala and its reversion by a benzodiazepine”. Neuropsychopharmacology 2021. https://doi.org/10.1038/s41386-021-01176-2
Froemke, … Jung, et al. (2021). “Neural circuitry for maternal oxytocin release induced by infant cries”. Research Square. https://doi.org/10.21203/rs.3.rs-970204/v1
Kutlu, … Calipari, et al. (2021). “Dopamine release in the nucleus accumbens core signals perceived saliency”. Current Biology, 31(21). https://doi.org/10.1016/j.cub.2021.08.052
Goldstein, … Alhadeff, et al. (2021). “Hypothalamic detection of macronutrients via multiple gut-brain pathways”. Cell Metabolism, 33(3). https://doi.org/10.1016/j.cmet.2020.12.018
Spring, … Wheeler, et al. (2021). “Chronic Stress Prevents Cortico-Accumbens Cue Encoding and Alters Conditioned Approach”. The Journal of Neuroscience, 41(11). https://doi.org/10.1523/JNEUROSCI.1869-20.2021
Dong, … Williams, et al. (2021). “Time and metabolic state-dependent effects of GLP-1R agonists on NPY/AgRP and POMC neuronal activity in vivo”. Molecular Metabolism, 54. https://doi.org/10.1016/j.molmet.2021.101352
Park, … Kim, et al. (2021). “Social isolation impairs the prefrontal-nucleus accumbens circuit subserving social recognition in mice”. Cell Reports, 35(6). https://doi.org/10.1016/j.celrep.2021.109104
He, … Xu, et al. (2021). “Barbadin Potentiates Long-Term Effects of Lorcaserin on POMC Neurons and Weight Loss”. The Journal of Neuroscience, 41(26). https://doi.org/10.1523/JNEUROSCI.3210-20.2021
Norman, … Morishita, et al. (2021). “Post-error recruitment of frontal sensory cortical projections promotes attention in mice”. Neuron, 109. https://doi.org/10.1016/j.neuron.2021.02.001
Sherathiya, … Lerner, et al. (2021). “GuPPy, a Python toolbox for the analysis of fiber photometry data”. Scientific Reports, 11(1). https://doi.org/10.1038/s41598-021-03626-9
de Kloet … Mansvelder, et al “Bi-directional regulation of cognitive control by distinct prefrontal cortical output neurons to thalamus and striatum”. Nature Communications Mar, 2021. doi: 10.1038/s41467-021-22260-7
Stern … Friedman, et al “Top-down control of conditioned overconsumption is mediated by insular cortex Nos1 neurons”. Cell Metabolism Mar, 2021. doi: 10.1016/j.cmet.2021.03.001
Peters … McCutcheon, et al “Distracting stimuli evoke ventral tegmental area responses in rats during ongoing saccharin consumption”. European Journal of Neuroscience Mar, 2021. doi: 10.1111/ejn.15108
Sathyanesan … Gallo, et al “Disruption of neonatal Purkinje cell function underlies injury-related learning deficits”. Proceedings of the National Academy of Sciences Mar, 2021. doi: 10.1073/pnas.2017876118
Bruno … Barker, et al “pMAT: An open-source software suite for the analysis of fiber photometry data”. Pharmacology Biochemistry and Behavior Feb, 2021. doi: 10.1016/j.pbb.2020.173093
Mayer … Blakely, et al “There’s no place like home? Return to the home cage triggers dopamine release in the mouse nucleus accumbens”. Neurochemistry International Jan, 2021. doi: 10.1016/j.neuint.2020.104894
Karigo … Anderson, et al “Distinct hypothalamic control of same- and opposite-sex mounting behaviour in mice”. Nature Jan, 2021. doi: 10.1038/s41586-020-2995-0
2020
Seiler, … Lerner, et al. (2020). “Dopamine signaling in the dorsomedial striatum promotes compulsive behavior”. BioRxiv. https://doi.org/10.1101/2020.03.30.016238
Alabi, … Fuccillo, et al. (2020). “Disruption of Nrxn1α within excitatory forebrain circuits drives value-based dysfunction”. ELife, 9. https://doi.org/10.7554/eLife.54838
Sofia Beas … Penzo, et al “A ventrolateral medulla-midline thalamic circuit for hypoglycemic feeding”. Nature Communications Dec, 2020. doi: 10.1038/s41467-020-19980-7
He … Xu, et al “Estrogen receptor-α expressing neurons in the ventrolateral VMH regulate glucose balance”. Nature Communications Dec, 2020. doi: 10.1038/s41467-020-15982-7
Hsu … Roitman, et al “Thirst recruits phasic dopamine signaling through subfornical organ neurons”. Proceedings of the National Academy of Sciences Dec, 2020. doi: 10.1073/pnas.2009233117
Bicks … Morishita, et al “Prefrontal parvalbumin interneurons require juvenile social experience to establish adult social behavior”. Nature Communications Dec, 2020. doi: 10.1038/s41467-020-14740-z
Jaramillo … Centanni, et al “BNST transient activity associates with approach behavior in a stressful environment and is modulated by the parabrachial nucleus”. Neurobiology of Stress Nov, 2020. doi: 10.1016/j.ynstr.2020.100247
Miletta … Horvath, et al “AgRP neurons control compulsive exercise and survival in an activity-based anorexia model”. Nature Metabolism Nov, 2020. doi: 10.1038/s42255-020-00300-8
Yamamuro … Morishita, et al “A prefrontal–paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice”. Nature Neuroscience Oct, 2020. doi: 10.1038/s41593-020-0695-6
Root … Morales, et al “Distinct Signaling by Ventral Tegmental Area Glutamate, GABA, and Combinatorial Glutamate-GABA Neurons in Motivated Behavior”. Cell Reports Sep, 2020. doi: 10.1016/j.celrep.2020.108094
Liu … McNally, et al “The Mesolimbic Dopamine Activity Signatures of Relapse to Alcohol-Seeking”. The Journal of Neuroscience Aug, 2020. doi: 10.1523/jneurosci.0724-20.2020
Barbano … Morales, et al “VTA Glutamatergic Neurons Mediate Innate Defensive Behaviors”. Neuron Jul, 2020. doi: 10.1016/j.neuron.2020.04.024
Cho … Sohal, et al “Cross-hemispheric gamma synchrony between prefrontal parvalbumin interneurons supports behavioral adaptation during rule shift learning”. Nature Neuroscience Jul, 2020. doi: 10.1038/s41593-020-0647-1
Gadziola … Wesson, et al “A Neural System that Represents the Association of Odors with Rewarded Outcomes and Promotes Behavioral Engagement”. Cell Reports Jul, 2020. doi: 10.1016/j.celrep.2020.107919
Jacobs, Moghaddam “Prefrontal Cortex Representation of Learning of Punishment Probability During Reward-Motivated Actions”. The Journal of Neuroscience Jun, 2020. doi: 10.1523/jneurosci.0310-20.2020
Steinberg … Malenka, et al “Amygdala-Midbrain Connections Modulate Appetitive and Aversive Learning”. Neuron Jun, 2020. doi: 10.1016/j.neuron.2020.03.016
Salimando … Winder, et al “BNST GluN2D-Containing NMDA Receptors Influence Anxiety- and Depressive-like Behaviors and ModulateCell-Specific Excitatory/Inhibitory Synaptic Balance”. The Journal of Neuroscience May, 2020. doi: 10.1523/jneurosci.0270-20.2020
Tan … Zuker, et al “The gut–brain axis mediates sugar preference”. Nature Apr, 2020. doi: 10.1038/s41586-020-2199-7
Berland … Luquet, et al “Circulating Triglycerides Gate Dopamine-Associated Behaviors through DRD2-Expressing Neurons”. Cell Metabolism Apr, 2020. doi: 10.1016/j.cmet.2020.02.010
Lafferty … Britt, et al “Nucleus Accumbens Cell Type- and Input-Specific Suppression of Unproductive Reward Seeking”. Cell Reports Mar, 2020. doi: 10.1016/j.celrep.2020.02.095
Pignatelli … Bonci, et al “Cooperative synaptic and intrinsic plasticity in a disynaptic limbic circuit drive stress-induced anhedonia and passive coping in mice”. Molecular Psychiatry Mar, 2020. doi: 10.1038/s41380-020-0686-8
Corkrum … Araque, et al “Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity”. Neuron Mar, 2020. doi: 10.1016/j.neuron.2019.12.026
Konanur … Roitman, et al “Phasic dopamine responses to a food-predictive cue are suppressed by the glucagon-like peptide-1 receptor agonist Exendin-4”. Physiology & Behavior Mar, 2020. doi: 10.1016/j.physbeh.2019.112771
Marcus … Patel, et al “Endocannabinoid Signaling Collapse Mediates Stress-Induced Amygdalo-Cortical Strengthening”. Neuron Mar, 2020. doi: 10.1016/j.neuron.2019.12.024
Gao … Penzo, et al “Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus”. Nature Neuroscience Feb, 2020. doi: 10.1038/s41593-019-0572-3
Salimando … Winder, et al “BNST GluN2D-Containing NMDA Receptors Influence Anxiety-and Depressive-like Behaviors and Modulate Cell-Specific Excitatory/Inhibitory Synaptic Balance”. The Journal of Neuroscience , 2020. doi: 10.1523/jneurosci.0270-20.2020
2019
Heifets … Malenka, et al “Distinct neural mechanisms for the prosocial and rewarding properties of MDMA”. Science Translational Medicine Dec, 2019. doi: 10.1126/scitranslmed.aaw6435
Mendoza … Britt, et al “Cue-Evoked Dopamine Neuron Activity Helps Maintain but Does Not Encode Expected Value”. Cell Reports Nov, 2019. doi: 10.1016/j.celrep.2019.09.077
Alhadeff … Betley, et al “Natural and Drug Rewards Engage Distinct Pathways that Converge on Coordinated Hypothalamic and Reward Circuits”. Neuron Sep, 2019. doi: 10.1016/j.neuron.2019.05.050
Shi … Fu, et al “A Rare Mutation of β1-Adrenergic Receptor Affects Sleep/Wake Behaviors”. Neuron Sep, 2019. doi: 10.1016/j.neuron.2019.07.026
Sengupta, Holmes “A Discrete Dorsal Raphe to Basal Amygdala 5-HT Circuit Calibrates Aversive Memory”. Neuron Aug, 2019. doi: 10.1016/j.neuron.2019.05.029
Guo … Polley, et al “The Cholinergic Basal Forebrain Links Auditory Stimuli with Delayed Reinforcement to Support Learning”. Neuron Jul, 2019. doi: 10.1016/j.neuron.2019.06.024
Parker … Bruchas, et al “A Paranigral VTA Nociceptin Circuit that Constrains Motivation for Reward”. Cell Jul, 2019. doi: 10.1016/j.cell.2019.06.034
Choi … McNally, et al “Paraventricular Thalamus Controls Behavior during Motivational Conflict”. The Journal of Neuroscience Jun, 2019. doi: 10.1523/jneurosci.2480-18.2019
Feng … Li, et al “A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine”. Neuron May, 2019. doi: 10.1016/j.neuron.2019.02.037
Cameron … Witten, et al “Increased Cocaine Motivation Is Associated with Degraded Spatial and Temporal Representations in IL-NAc Neurons”. Neuron May, 2019. doi: 10.1016/j.neuron.2019.04.015
Mansy … Oweiss, et al “Spatial detection characteristics of a single photon fiber photometry system for imaging neural ensembles *”. 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER) Mar, 2019. doi: 10.1109/ner.2019.8717005
Zimmer … Dietrich, et al “Functional Ontogeny of Hypothalamic Agrp Neurons in Neonatal Mouse Behaviors”. Cell , 2019. doi: 10.1016/j.cell.2019.04.026
Holly … Fuccillo, et al “Striatal Low-Threshold Spiking Interneurons Regulate Goal-Directed Learning”. Neuron , 2019. doi: 10.1016/j.neuron.2019.04.016
Li … Krashes, et al “Defined Paraventricular Hypothalamic Populations Exhibit Differential Responses to Food Contingent on Caloric State”. Cell Metabolism , 2019. doi: 10.1016/j.cmet.2018.10.016
Robinson … Gradinaru, et al “Optical dopamine monitoring with dLight1 reveals mesolimbic phenotypes in a mouse model of neurofibromatosis type 1”. eLife , 2019. doi: 10.7554/elife.48983
Bai … Knight, et al “Genetic Identification of Vagal Sensory Neurons That Control Feeding.”. Cell , 2019. doi: 10.1016/j.cell.2019.10.031
2018
Pascoli … Lüscher, et al “Stochastic synaptic plasticity underlying compulsion in a model of addiction”. Nature Dec, 2018. doi: 10.1038/s41586-018-0789-4
Reed … Britt, et al “Coordinated Reductions in Excitatory Input to the Nucleus Accumbens Underlie Food Consumption”. Neuron Sep, 2018. doi: 10.1016/j.neuron.2018.07.051
Walsh … Malenka, et al “5-HT release in nucleus accumbens rescues social deficits in mouse autism model”. Nature Aug, 2018. doi: 10.1038/s41586-018-0416-4
Saunders … Janak, et al “Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties”. Nature Neuroscience Jul, 2018. doi: 10.1038/s41593-018-0191-4
Giza … Lee, et al “The BDNF Val66Met Prodomain Disassembles Dendritic Spines Altering Fear Extinction Circuitry and Behavior”. Neuron Jul, 2018. doi: 10.1016/j.neuron.2018.05.024
Beas … Penzo, et al “The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism”. Nature Neuroscience Jun, 2018. doi: 10.1038/s41593-018-0167-4
Fang … Lin, et al “A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors”. Neuron Apr, 2018. doi: 10.1016/j.neuron.2018.02.019
Sengupta … McNally, et al “Basolateral Amygdala Neurons Maintain Aversive Emotional Salience”. The Journal of Neuroscience Mar, 2018. doi: 10.1523/jneurosci.2460-17.2017
Augustine … Oka, et al “Hierarchical neural architecture underlying thirst regulation”. Nature Mar, 2018. doi: 10.1038/nature25488
Wang … Krauzlis, et al “Activation of Striatal Neurons Causes a Perceptual Decision Bias during Visual Change Detection in Mice”. Neuron Mar, 2018. doi: 10.1016/j.neuron.2018.01.049
Chen … Dan, et al “A Hypothalamic Switch for REM and Non-REM Sleep”. Neuron Mar, 2018. doi: 10.1016/j.neuron.2018.02.005
2017
Hashikawa … Lin, et al “Esr1+ cells in the ventromedial hypothalamus control female aggression”. Nature Neuroscience Nov, 2017. doi: 10.1038/nn.4644
Barker … Morales, et al “Lateral Preoptic Control of the Lateral Habenula through Convergent Glutamate and GABA Transmission”. Cell Reports Nov, 2017. doi: 10.1016/j.celrep.2017.10.066
Calipari … Nestler, et al “Dopaminergic dynamics underlying sex-specific cocaine reward”. Nature Communications Apr, 2017. doi: 10.1038/ncomms13877
2016
Wells … Halassa, et al “Thalamic reticular impairment underlies attention deficit in Ptchd1Y/− mice”. Nature Mar, 2016. doi: 10.1038/nature17427
Zalocusky … Deisseroth, et al “Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making”. Nature Mar, 2016. doi: 10.1038/nature17400
Calipari … Nestler, et al “In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward”. Proceedings of the National Academy of Sciences of the United States of America , 2016. doi: 10.1073/pnas.1521238113
2015
Wimmer … Halassa, et al “Thalamic control of sensory selection in divided attention”. Nature Oct, 2015. doi: 10.1038/nature15398